The Israeli MedTech startup is riding the wave of remote medical solutions, which have gained even more popularity since the outbreak of the COVID-19 pandemic.
MyHomeDoc, an Israeli startup developing a remote diagnosis device, has received an FDA approval for its telehealth solution. The FDA OK was received before opening the company to the coveted U.S. healthcare market.
Backing from Phillips and Teva
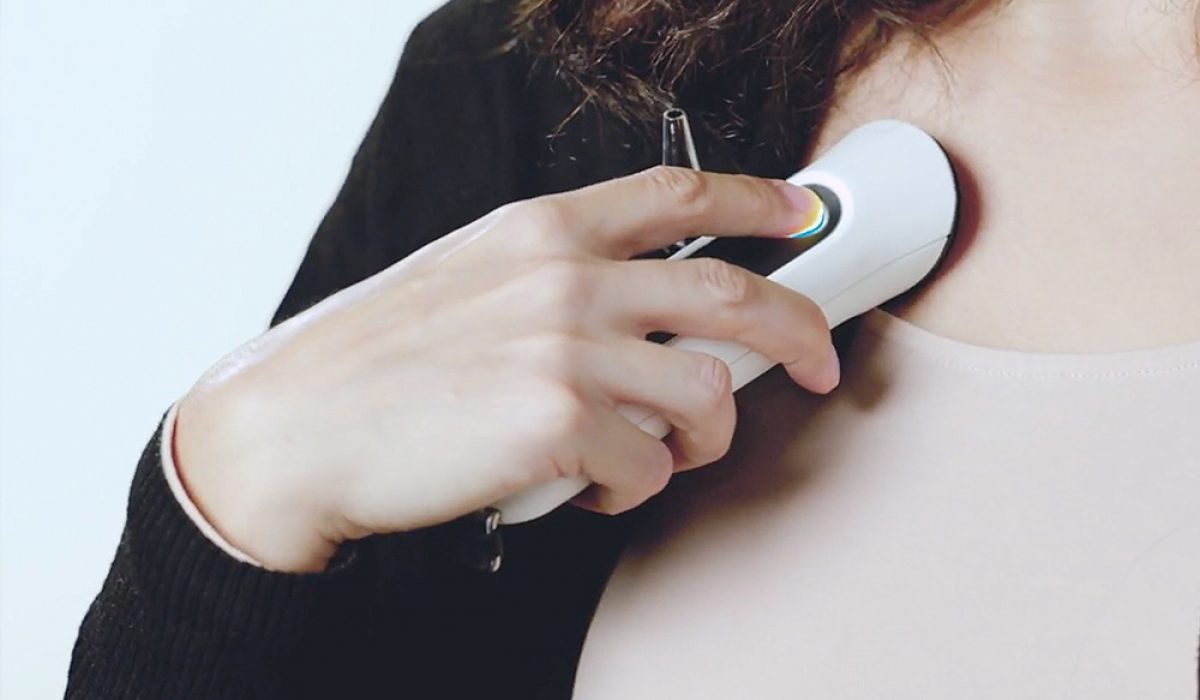
The device developed by MyHomeDoc relies on 4 embedded sensors which connect with the user’s smartphone to provide instant remote vital signs readings, by performing the nine most common tests required for primary medical care. The 9 tests include a stethoscope check (lung, heart, and bowel sounds), otoscopy of the ear, oximeter (pulse rate and saturation), thermometer (body temperature), and a throat and skin test that uses the smartphone’s camera. The MyHomeDoc allows patients from anywhere and at any time to send vital sign readings directly to the user’s caring physician, who can prioritize his checkups accordingly.
Furthermore, the device, which can be used on children as young as two years old, also helps reduce frequent clinic visits for elderly, patients with preexisting conditions, chronic pain, or others, becoming even more prevalent during the social distancing pandemic. Users are treated with an easy-to-use interface where they can receive their doctor’s review, referral, or prescription in real-time.